 Home -
Products -
Angiogenesis -
CDK -
Benzo[b]thiophene-2-sulfonamide, N-[3-(aminomethyl)phenyl]-5-chloro-3-methyl-
Home -
Products -
Angiogenesis -
CDK -
Benzo[b]thiophene-2-sulfonamide, N-[3-(aminomethyl)phenyl]-5-chloro-3-methyl-
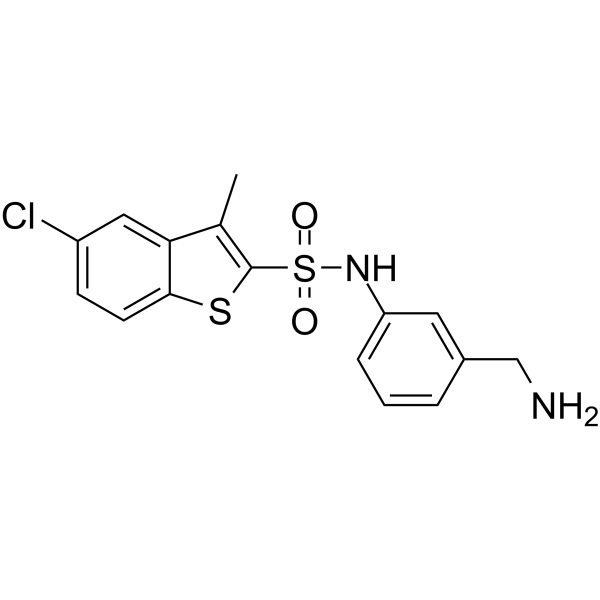
Benzo[b]thiophene-2-sulfonamide, N-[3-(aminomethyl)phenyl]-5-chloro-3-methyl-
CAS No. 2428737-43-1
Benzo[b]thiophene-2-sulfonamide, N-[3-(aminomethyl)phenyl]-5-chloro-3-methyl-( —— )
Catalog No. M28870 CAS No. 2428737-43-1
Benzo[b]thiophene-2-sulfonamide, N-[3-(aminomethyl)phenyl]-5-chloro-3-methyl- directly inhibits InhA and does not require activation by the catalase peroxidase KatG.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 302 | Get Quote |


|
| 10MG | 447 | Get Quote |


|
| 25MG | 714 | Get Quote |


|
| 50MG | 1017 | Get Quote |


|
| 100MG | 1368 | Get Quote |


|
| 500MG | 2673 | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameBenzo[b]thiophene-2-sulfonamide, N-[3-(aminomethyl)phenyl]-5-chloro-3-methyl-
-
NoteResearch use only, not for human use.
-
Brief DescriptionBenzo[b]thiophene-2-sulfonamide, N-[3-(aminomethyl)phenyl]-5-chloro-3-methyl- directly inhibits InhA and does not require activation by the catalase peroxidase KatG.
-
DescriptionBenzo[b]thiophene-2-sulfonamide, N-[3-(aminomethyl)phenyl]-5-chloro-3-methyl- directly inhibits InhA and does not require activation by the catalase peroxidase KatG.
-
In VitroInhA-IN-2 (Compound 23) (200 μM, 48h) inhibits mycolic acid synthesis (33% growth inhibition) in M. tuberculosis H37Ra.
-
In Vivo——
-
Synonyms——
-
PathwayAngiogenesis
-
TargetCDK
-
RecptorCytochrome P450
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number2428737-43-1
-
Formula Weight366.89
-
Molecular FormulaC16H15ClN2O2S2
-
Purity>98% (HPLC)
-
Solubility——
-
SMILESCc(c(cc(cc1)Cl)c1s1)c1S(Nc1cc(CN)ccc1)(=O)=O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Kasem Nithipatikom, et al. Inhibition of carcinoma cell motility by epoxyeicosatrienoic acid (EET) antagonists. Cancer Sci. 2010 Dec;101(12):2629-36.
molnova catalog



related products
-
Purvalanol B
A potent, cell-permeable, selective inhibitor of CDK with IC50s of 6, 6, 9, and 6 nM for cdc2/cyclin B, cdk2/cyclin A, cdk2/cyclin E, and cdk5-p35 respectively.
-
Purvalanol A
A potent, cell-permeable, selective inhibitor of CDK with IC50s of 4, 70, 35, 850, and 75 nM for cdc2/cyclin B, Cdk2/cyclin A, Cdk2/cyclin E, Cdk4/cyclin D1 and Cdk5-p35, respectively.
-
Dalpiciclib
Dalpiciclib is a highly selective, orally bioavailable, and comparable potency inhibitor of CDK4 and CKD6 with IC50s of 12.4?nM and 9.9?nM, respectively.



 Cart
Cart
 sales@molnova.com
sales@molnova.com

